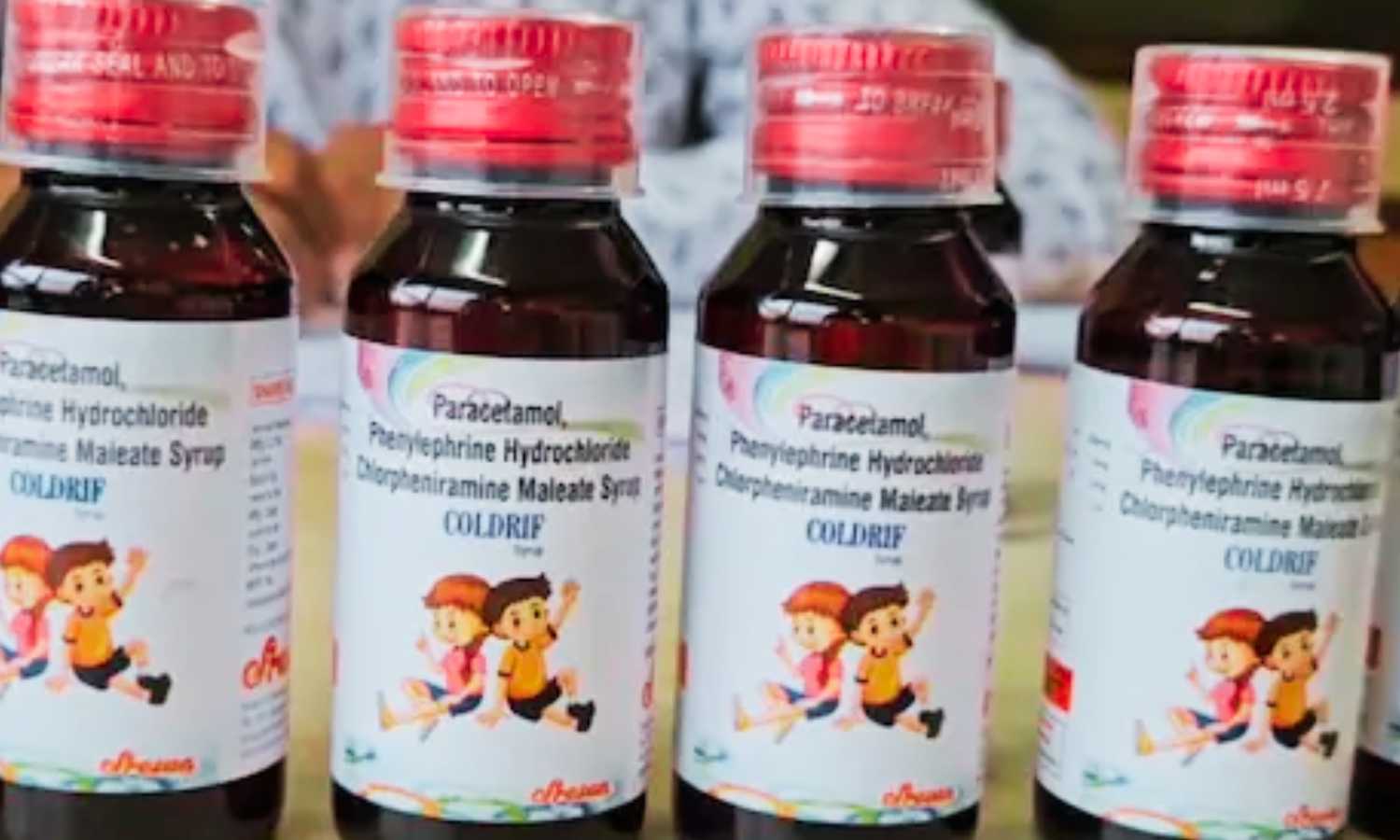
New Delhi: After Tamil Nadu, the governments of Madhya Pradesh and Kerala also banned the Coldrif cough syrup following reports of child deaths linked to the medicine. In Madhya Pradesh, 10 children aged between one and five died within 27 days after consuming the syrup.
The syrup originated from a manufacturing unit in Kanchipuram, Tamil Nadu. Consequently, the Tamil Nadu government banned its production and sale on Thursday and seized stocks from wholesale and retail outlets.
In Madhya Pradesh, all deaths occurred in Chhindwara district. The first suspected case appeared on August 24, and the first confirmed death followed on September 7. Over the next 15 days, six more children died of kidney failure. State Food and Drug Controller Dinesh Kumar Maurya explained that tests showed excessive diethylene glycol, which rendered the syrup toxic.
CDSCO begins pan-India drug plant inspections
Meanwhile, the Union Health Ministry announced that the Central Drugs Standard Control Organisation (CDSCO) launched inspections across six states on October 3. Altogether, 19 pharmaceutical plants producing cough syrups and antibiotics came under review to check for quality control lapses.
In Rajasthan, two more child deaths were linked to Dextromethorphan Hydrobromide syrup produced by Jaipur-based Kasons Pharma. Therefore, the Rajasthan government banned all 19 drugs made by the company. It also suspended Drug Controller Rajaram Sharma for negligence since he had earlier cleared the firm in a probe.
In addition, the Union Health Ministry issued a national advisory. It urged parents not to give cough syrups to children under two years. Furthermore, the DGHS advised that such medicines should be used cautiously even for children under five, under strict medical supervision and with limited dosage.